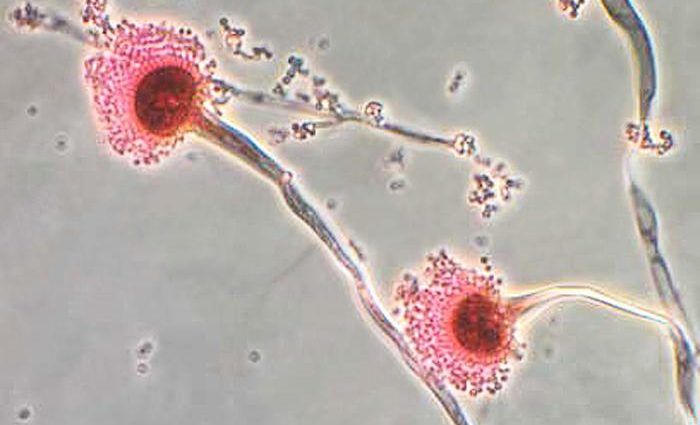
On November 2, 2021, the Florida Department of Health’s Office of Medical Marijuana Use (OMMU), determined that Curaleaf Florida, LLC d/b/a Curaleaf (Curaleaf) dispensed medical marijuana products to qualified patients that reported a failed testing value of Aspergillus in violation of Rule 64ER20-9(2)(a)3., Florida Administrative Code.
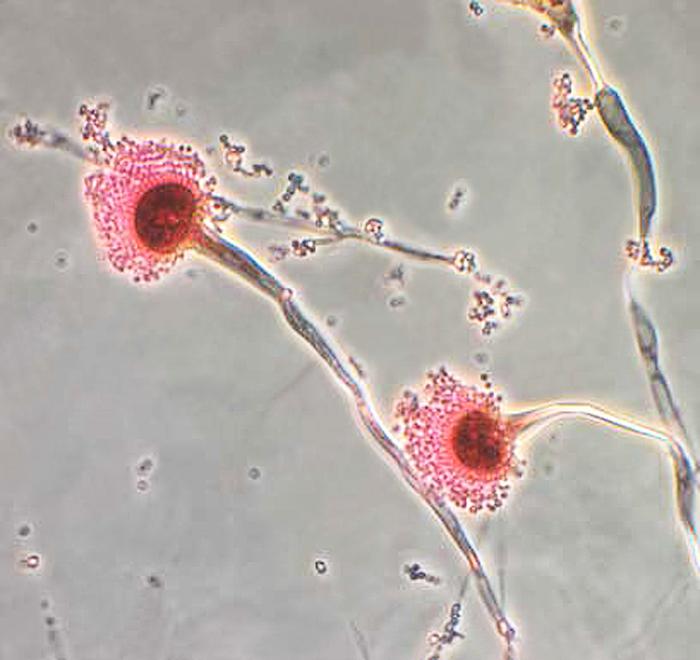
The contaminated products, Lilac Diesel Mini Buds (#TLMB0716202103) and Pre-Pack FL PWP (#TLF0728202102), should not be consumed. The OMMU required Curaleaf to immediately recall all contaminated product and notify all patients and caregivers that received the product.
Per the Centers for Disease Control and Prevention (CDC), Aspergillosis is an infection caused by Aspergillus, a common mold (a type of fungus) that lives indoors and outdoors. Most people breathe in Aspergillus spores every day without getting sick. However, people with weakened immune systems or lung diseases are at a higher risk of developing health problems due to Aspergillus. The types of health problems caused by Aspergillus include allergic reactions, lung infections and infections in other organs.
The OMMU is charged with regulating medical marijuana in Florida to ensure patient safety. The office’s responsibilities include writing and implementing the Department’s rules for medical marijuana, overseeing the statewide Medical Marijuana Use Registry, and licensing Medical Marijuana Treatment Centers (MMTCs) to cultivate, process and dispense medical marijuana to qualified patients.
CPAP RECALL: FDA UPDATE ON CERTAIN PHILIPS RESPIRONICS BREATHING ASSISTANCE MACHINES
DOLE SALAD RECALLED DUE TO LISTERIA RISK, DISTRIBUTED IN FLORIDA